We’re proud to announce two major milestones! 🎉
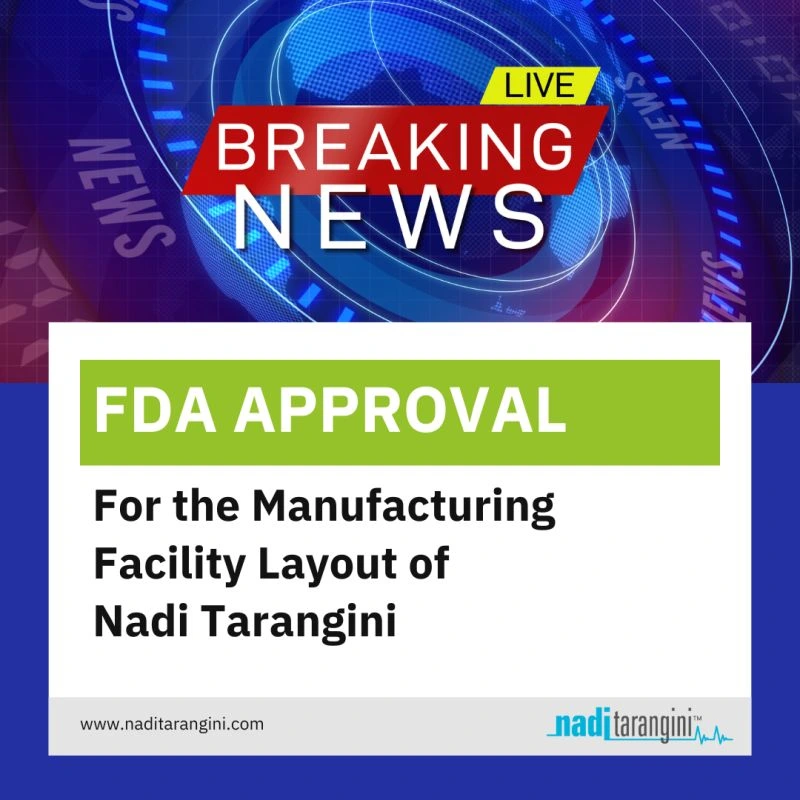
Our Pune-based Nadi Tarangini manufacturing facility has received the FDA layout approval, ensuring we meet top regulatory standards for medical device production. Bringing us one step closer to delivering innovative #AyurTech solutions. This milestone reinforces our commitment to developing high-quality, innovative solutions that advance personalized healthcare.
Additionally, we’ve achieved GDPR compliance, ensuring our data and APIs adhere to stringent EU privacy regulations, furthering our commitment to data security and transparency as we expand into the European market. Achieving GDPR compliance means we are fully aligned with European Union data protection regulations, ensuring that personal information is collected, stored, and processed securely and ethically. With stringent data protection measures in place, we’re proud to confidently engage with clients and partners across the European market, ensuring that user privacy and security remain top priorities.
We’re also thrilled to share that we’ve received ISO 13485 and IEC 60601-1 certifications, reinforcing our focus on quality, safety, and innovation. The ISO 13485 certification ensures that our quality management system meets the rigorous standards required for medical devices , emphasizing our commitment to producing safe and effective products. And, the IEC 60601-1 certification demonstrates our adherence to international safety and performance standards for medical electrical equipment.
Here’s to more success ahead! 🚀
Atreya Innovations Private Limited
Patented Nadi Sensor
🌐 Nadi Tarangini
✉️ info@atreyainnovations.com
📞 +91-7774040185
